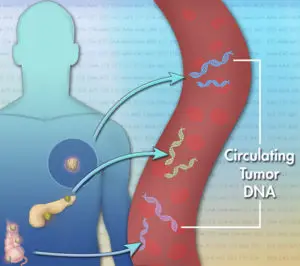
Each cancer is unique at the molecular level. A genomic profiling test can reveal clinically relevant alterations in DNA that drive cancer growth to help identify personalized treatment options based on a tumor’s unique genomic profile. Liquid biopsy is a simple and non-invasive alternative to surgical biopsies which enables doctors to discover a range of information about a tumor through a simple blood sample. Traces of the cancer’s DNA in the blood can give clues about which treatments are most likely to work for that patient. The non-invasive liquid biopsies, which require only 5 millilitres of blood, are quicker and much better to tolerate compared to a surgical biopsy.
The biggest benefit lies in the potential of liquid biopsies to detect disease progression or treatment resistance before it would trigger clinical symptoms or appear on imaging scans.
Most cancers have multiple genetic mutations and they may not have the same ones in all parts of the cancer. The tissue samples removed for biopsy may not show all mutations whereas liquid biopsies offer an improved chance of detecting genetic heterogeneity.